Finding Oxidation Numbers Worksheet Answers. For example to find the oxidation state of sulfur in h 2 so 4 h 2 so 4 h 2 1 2 o 4 2 8 6 s. The oxidation number of any uncombined element is 0 the oxidation number of a monatomic ion equals the charge on the ion.
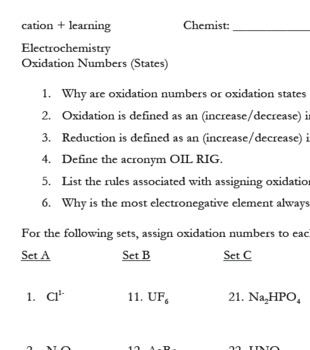
6 0 overall charge 6 find the oxidation state of each of the following 1. The sum of the oxidation number of all the elements in a compound equals 0. Se in h 2 seo 3.
Oxygen has an oxidation number of 2 unless it is combined with f when it is 2 or it is in a.
In this video you ll be presented with nine practice problems that become increasi. Terms in this set 20 1 4 2. The oxidation number of fluorine in a compound is always 1. Worksheet will open in a new window.
